∧ Back to top3. Experimental
From the non-uniformity of the visual parameters of the glass fragments encountered at a given archaeological site (colour, thickness, deterioration and surface texture) also a variation in the chemical composition can be expected. First, the fragments were classified on the basis of their visual outlook into approximately homogeneous groups. Per group, only one sample was retained for sampling and analysis in order to avoid the sampling of two fragments originating from the same pane. The samples were dated by their archaeological context. Fragments were sampled from different sites in Antwerp and from different other cities in Belgium. The analysed set encompasses a large number of plain window glass fragments, excavated stained-glass fragments, and fragments originating from intact windows. Most of the glass originated from secular buildings, but the analysed series also included a small number of glass fragments originating from churches, chapels or monasteries. The medieval glass in the analysed series is somewhat underrepresented because it was much harder to find than 16-17th century glass. Except for a couple of blue and red flashed glass fragments, all samples were made in naturally coloured glass with a light green hue. Additionally, the analysed set contained 3 fragments of 15-17th century mirror glass excavated in Antwerp.
Several glass splinters were embedded in the same block of resin. The orientation of the splinters in the resin was such that a cross-section, perpendicular to the original glass surface, could be studied. The surface of the resin was ground flat with corundum papers and polished with fine diamond pastes (down to a final diameter of 1 µm for the diamond powder). A carbon coating was applied to the polished surface. The samples were analysed by means of a JEOL 6300 electron microprobe system equipped with a digital, thin-window energy dispersive Si(Li) X-ray detector of Princeton Gamma Tech (PGT). This instrument is able to collect x-ray spectra of small areas of non-conducting material containing light elements (Na, Mg, Al, etc.). It is a suitable technique for major element compositional analysis of silicate materials.
From every glass sample, four x-ray spectra were collected at different positions using a voltage of 20 kV, a beam current of 2 nA, a 100 s acquisition time and a magnification of 500. A quantification algorithm based on thin film sensitivity coefficients was used to calculate the composition of the window glass (Schalm, 2000; Schalm and Janssens, 2003). Finally, after analysis of a series of glass fragments, a data matrix is obtained in which each row contains the average composition of a sample while each column corresponds to an oxide constituent of the glass (e.g., Na2O, CaO, etc.). In order to categorise the analysed glass fragments into compositionally similar groups, hierarchical clustering was performed on the data matrix by means of the software package SPSS. During the hierarchical clustering (Ward's method, squared Euclidian distance, concentration values standardised to a range of 0-1) the concentrations of Na2O, MgO, SiO2, K2O and CaO (expressed in weight %) were employed in order to subdivide the analysed set into nine clusters of glass fragments with significantly different compositions.
∧ Back to top4. Results
In the literature (Henderson, 1988; Wedepohl, 1997), a distinction is often made between lead glass (SiO2-K2O-CaO-PbO), soda glass (Na2O-SiO2-CaO), mixed-alkali glass (Na2O-SiO2-K2O-CaO), potash or forest glass (SiO2-K2O-CaO) and high-lime low-alkali (HLLA) glass (SiO2-K2O-CaO). The group of potash glass and HLLA glass is sometimes collectively denoted as calco-potassic glass. However, the relation between these names and the chemical composition is not always explicitly mentioned in all studies. To facilitate the discussion provided below, a taxonomy of compositional glass types is proposed in Fig. 4. This hierarchical ordering of glass types coincides with published classifications (see, e.g. , Gratuze, 1994; Müller et al., 1994; Barrera and Velde, 1989).
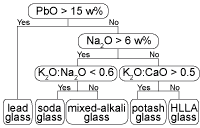
Fig. 4: Hierarchical classification of window glass fragments based on their major composition used in this investigation.
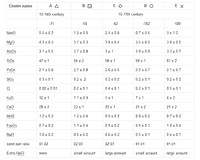
Table 1a: Average composition (in % w/w) of 434 HLLA glass samples; Table 1b: Average composition (in % w/w) of 47 potash glass samples.
The above-mentioned glass types usually are associated with different glassmaking recipes. Therefore, soda glass, potash glass and HLLA glass are sometimes described as soda-lime glass, wood ash glass and wood ash-lime glass respectively. Due to a large compositional variation within each type, a clear distinction between these types is not always straightforward. For example, glass fragments can contain a small amount of PbO, sometimes up to 7%, but this is not sufficient to classify them as lead glass. Lead glass contains in many cases between 30 and 60% PbO. It should be remarked that some glass compositions are close to the selection criteria mentioned in Fig. 4, for example those with a K2O:CaO ratio of 0.509. Therefore, a classification of individual samples with such a flow chart is not recommended. Instead, hierarchical clustering was first employed to classify the samples into clusters and only in a second step, the average compositions of these clusters were associated with a certain glass type by means of the flow chart given in Fig. 4. All samples in such a cluster then become associated with the glass type that corresponds best to the average cluster composition. If necessary, clusters of the same glass type can be grouped together. The average composition of the resulting nine clusters is listed in Table I.
The classification of the data set into nine compositional clusters resulted in five HLLA glass subtypes, three potash glass subtypes and one soda glass type. The five soda glass fragments encountered are respectively 16-17th century mirrors excavated in Antwerp (three pieces), a colourless oval-shaped pane (one piece) and a 19th century restoration in an older stained-glass window (one piece); therefore, they stand apart from the series of window panes investigated here. All window glass samples that were analysed consisted of calco-potassic glass. No lead glass or soda glass was identified within this set of 12-18th century window glass fragments. Most 15-17th century non-figurative window glass fragments from secular buildings were made in HLLA glass (403 samples); only a limited number of potash glass fragments (26 samples) were encountered among the 486 analysed. The substrate glass of stained-glass windows from the 12-15th century was found to be either potash glass (21 samples) or HLLA glass (39 samples). All glass fragments sampled from 16-17th century painted glass widows turned out to be HLLA glass (71 samples).
Corresponding to the periods 12-14th century and 15-(17th?) century, a large group of HLLA glass and a smaller group of potash glass was identified by means of hierarchical cluster analysis. Potash glass appears to be more frequently present in 12-15th century stained-glass windows. Most of the 15-17th century glass is characterised by MgO concentrations higher than 5% w/w.
The scatter plot in Fig. 5 illustrates the large compositional variation within the clusters and the small distances that exist between the centres of these clusters. Therefore, it is not surprising that the membership of a sample to a specific cluster depends on the classification method that has been employed. However, clear differences between the average compositions of the clusters were observed. These averages describe the largest compositional variation in the data set and can be explained by the use of different glassmaking recipes or by the use of raw materials with a specific composition. The variation within the clusters is probably caused by compositional variations of the raw materials, by slight fluctuations of the relative amounts of ingredients in the recipe and by the introduction of cullet (recycled glass) with a different composition into the batch.
Most of what is known about medieval stained-glass manufacturing technology originates from the 12th century manuscript On Diverse Arts by Theophilus (Howthorne and Smith, 1963). According to this author, glass was made with one part of washed sand and two parts of beechwood ash (ibid. Book II, Chapter IV). The low amount of SiO2 in the 12-14th century window glass (< 50 w%) and the large quantities of oxides that have been introduced by the fluxing agent (K2O + CaO _ 40 w%) might suggest that this glass was indeed made by means of a recipe resembling that of Theophilus. The P2O5 concentration of about 2% w/w points to the use of unpurified wood ash. However, the presence of potash glass (K2O:CaO ratio of ca. 1) and HLLA glass (K2O:CaO ratio of 0.4) is evidence for the use of different recipes in that period or at least of the use of different raw materials used in combination with the same recipe.
From the end of the 14th century onwards several compositional changes can be observed.
- A change in SiO2-abundance. While the medieval glass (clusters A, F) contains less than 50% w/w SiO2, the window glass from the 15-17th century (clusters B-E, G) generally features a SiO2-concentration significantly above 50 w%. In the HLLA glass from the 15-17th century, several clusters showing different SiO2-concentrations (between 56 w% and 61 w%) could be identified, but it is not clear whether these groups existed next to each other or whether they are the results of a gradual compositional evolution. This higher SiO2-abundance undoubtedly must have been caused by the adoption of a recipe for glass making, involving with more sand and less fluxing agent(s). The average SiO2-abundances of the HLLA glass types correspond to experimentally reproduced glass with sand: ash ratios of respectively 2:3 (52-60 w%, clusters B,C) and 1:1 (62-66 w%, clusters D,E) (Stern and Gerber, 2004).
- A change in K2O:CaO ratio. Among the HLLA glass, this ratio varies from about 1:3 (clusters B, D) to about 1:5 (cluster E). This means that the chemical composition of 15-17th century HLLA glass could only be obtained when the wood ash employed in the medieval recipe was replaced by an ash richer in calcium or by a mixture of fluxing agents, for example wood ash and lime.
- Additional use of NaCl. The fluxing agent employed since the 15th century also contained more Na2O and less K2O. The medieval window glass (cluster A) features a somewhat lower concentration of Na2O than 15-17th century glass, although higher amounts of wood ash – the major source of alkali – were employed for its production. This suggests that an additional sodium source was employed for the fabrication of these 15-17th century glass. A correlation between Na2O and Cl, shown in Fig. 6, was only found within the group of 15-17th century HLLA glass (clusters B-D). The Na2O-concentration varies between 0 and 5% w/w, while the Cl-concentration varies between 0 and 0.8 w%. For all calco-potassic samples, the content of Na2O was smaller than 6 w%. Most probably, sea salt was employed as additional source of sodium. During glass fusion, this ingredient transforms into Na2O while most of the chlorine evaporates. Only in one sample the high Na2O content appears to be unrelated to the chlorine content. An occasional admixture of (Cl-poor) soda-glass cullet to the batch might explain this exception. The use of sea salt indicates that not only the sand: ash ratio in the glassmaking recipe changed at the end of the 14th century, but that also the use of new starting materials was introduced into the preparation procedure.
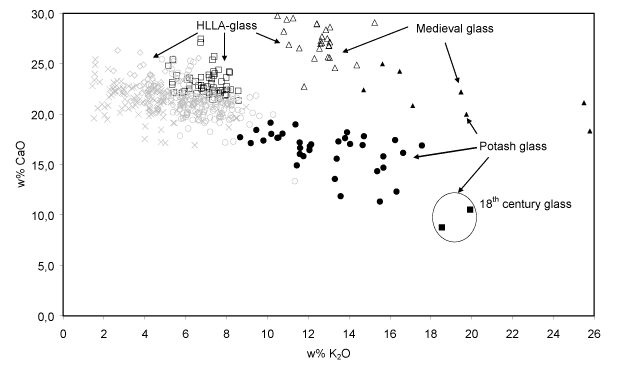
- Na2O-K2O and Cl (anti?)-correlation. Since on the one hand wood ash is poor in sodium (Misra et al., 1993; Stern and Gerber, 2004) while on the other hand sea salt is poor in potassium and calcium (Brown et al., 1991), no clear relation between the Na2O-abundance and that of other oxides such as K2O and CaO is to be expected when a mixture of sea salt and wood ash is used as a fluxing agent for glass production. Indeed, for the 15-17th century HLLA glass such no relation is found between Na2O and CaO: the concentration of CaO remains constant at around 22% w/w while the concentration of Na2O varies between 0.0 and 6.0% w/w. However, the abundance of Na2O and K2O appears to be anti-correlated: glass with richer in Na2O (and also richer in Cl) generally contains less K2O. This is shown in Fig. 7. This suggests that the relative amounts of starting materials rich in sodium and rich in potassium were not chosen independently.
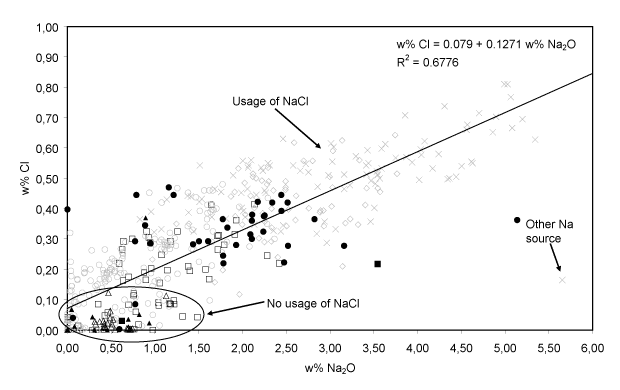
Fig. 6: The linear relation between Na2O and Cl for the 15-17th century HLLA glass. Most of the medieval glass does not follow this relation. The symbols are related to the compositional groups of Table I.
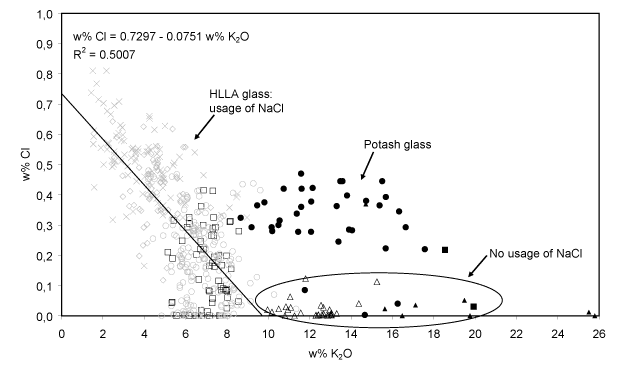
Fig. 7: The Cl-abundance as a function of K2O concentration for the calco-potassic glass fragments. For the 15-17th century HLLA glass (403 samples), an anti-correlation between these constituents clearly visible. Symbols: see Table I.
In contrast to the possibly gradual evolution in window glass composition during the 15-17th C., in the 18th century a new type of potash glass appears to have been introduced (two fragments in cluster H), featuring an even higher SiO2-abundance (67.3 5 w/w) than in previous periods and also characterised by low P2O5 concentrations. The latter probably indicate that for the manufacture of this type of glass, only the soluble part of wood ash was employed as starting material. This compositional change can only be explained by the introduction of a new recipe and the use of other flux material(s). However, in order to confirm this hypothesis a larger number of 18th century window glass fragments than two will need to be analysed.